For patients with suspected or confirmed Covid-19 who require intubation, a number of guidelines and reviews have recommended tracheal intubation is conducted with a video laryngoscope in preference to a direct laryngoscope.1,2,3
A video laryngoscope allows the clinician to view the anatomical structures on a screen or monitor, positioning them further away from the face of the patient than a direct laryngoscope, thereby potentially reducing the risk of exposure to patient respiratory aerosols and transmission of infection. 4,5
Videolaryngoscopy may also offer additional benefits to the clinician when intubating patients with Covid-19, where the objective is to achieve first-pass success and multiple attempts at intubation are likely to increase risk to both healthcare personnel and patients.1
A set of guidelines for managing the airway in patients with Covid-19 from the UK Difficult Airway Society, the Association of Anaesthetists, the Intensive Care Society, Royal College of Anaesthetists and the Faculty of Intensive Care Medicine, has been published in the journal Anaesthesia. In a section regarding the fundamentals of airway management for a patient with suspected or confirmed Covid-19, it is recommended to, ‘Use techniques that are known to work reliably across a range of patients, including when difficulty is encountered.’ The list includes videolaryngoscopy for tracheal intubation, and a 2nd generation supraglottic airway for airway rescue. For emergency tracheal intubation, the authors confirm that, ‘laryngoscopy should be undertaken with the device most likely to achieve prompt first-pass tracheal intubation in all circumstances in that operator’s hands – in most fully trained airway managers this is likely to be a video laryngoscope.’1
In a paper still in press at the time of writing, reporting data from a two-centre retrospective observational case series of 202 patients with coronavirus disease undergoing tracheal intubation in Wuhan, China, 10.4% of patients were intubated with a direct laryngoscope. The majority (89.6%) were intubated with a video laryngoscope. The data was analysed and discussed by a panel of international airway experts who produced a set of consensus recommendations included in the paper. These included videolaryngoscopy over direct laryngoscopy and head elevated positioning prior to intubation to optimise intubation conditions.2
In regard to resuscitation, The European Resuscitation Council Covid-19 Guidelines for Advanced Life Support in Adults, confirm that, ‘Experienced airway staff should insert a supraglottic airway or intubate the trachea early so that the period of bag-mask ventilation is minimised. Consider video-laryngoscopy for tracheal intubation by providers familiar with its use – this will enable the intubator to remain further from the patient’s mouth.’6
Whilst none of the above papers recommend a specific videolaryngoscope, it has been suggested in some articles there may be a benefit to devices with a separate screen. In one paper, this is recommended on the basis that it enables the user to stay further away from the airway1. However, whether this is correct has yet to be established, since the operator is still required to hold the video laryngoscope in place, limiting the distance they can be from the patient’s head during the procedure. A separate screen may also have disadvantages, particularly if it is not optimally positioned and may draw the attention of the operator away from the patient’s head if it is not located centrally. A separate screen also introduces additional components to the intubation room and, unless single use, will be another piece of equipment requiring some form of cleaning, disinfection and reprocessing prior to reuse.
Particularly important in the context of a discussion about airway management in patients with Covid-19 is the issue of single use versus reusable devices. Whilst the recent consensus guidelines published in Anaesthesia do not consider this question specifically in relation to video laryngoscopes, they do discuss the issue in more general terms, and confirm that, ‘where practical, single-use equipment should be used.’ Of course, there are caveats to this, particularly where quality may vary between devices and the authors qualify their statement, acknowledging that, ‘where single use equipment is not of the same quality as re-usable equipment this creates a conflict.’1
Interestingly, the Association of Anaesthetists produced a guidance document, entitled, ‘Guidelines – Infection prevention and control 2020’, (published before the Covid-19 outbreak) which does comment directly on video laryngoscopes in the context of infection control, confirming that, ‘Single-use video laryngoscopes minimise any chance of cross contamination and would be ideal, but many single-use video laryngoscopes have reusable components that need to be decontaminated after each use.7 This is correct, since many devices incorporate single use blades, but still have reusable screens, monitors and handles. However, a completely single use and fully integrated disposable video laryngoscope is available to the clinician.
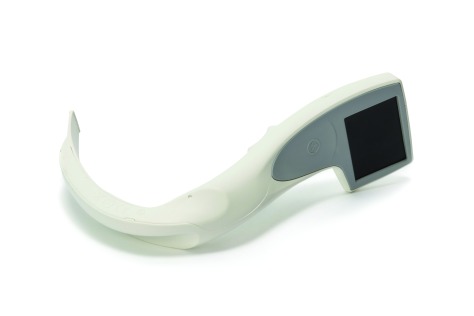
The i-view™ is a single use, fully disposable video laryngoscope from Intersurgical and offers the clinician the only adult, one size, single use, disposable and fully integrated video laryngoscope with a Macintosh blade.8 It requires no accessory products such as external monitors, cables or power source. As a result, it minimises any chance of cross contamination and eliminates concern related to the cost, availability or effectiveness of appropriate methods of decontamination and reprocessing.

In addition to the infection control benefits, i-view™ also offers the opportunity for videolaryngoscopy wherever and whenever the clinician needs to intubate. By incorporating a Macintosh blade, i-view™ can also be used for direct as well as videolaryngoscopy. Where availability of a video laryngoscope may be limited due to the cost implications of purchasing reusable devices for multiple sites, i-view™ provides a cost effective solution, by combining all the advantages of a fully integrated video laryngoscope in a single use, disposable product. This makes i-view™ ideal for use in resuscitation, pre-hospital, emergency medicine, intensive care as well as for difficult airway cases and anaesthesia.
Whilst there are not yet, to our knowledge, any robust randomised controlled trials (RCTs) evaluating and comparing the performance of different video laryngoscopes during the current pandemic, particularly when wearing Personal Protective Equipment (PPE), a recent RCT published in April assessed visual grading of the glottis and hemodynamic response to laryngoscopy for the i-view™ compared to another video laryngoscope in patients with a BMI >50kg/m2. This concluded that both devices allowed for safe and effective intubation in this patient group. This study was conducted before the current pandemic and without PPE.
As highlighted in the consensus guidelines for managing the airway in patients with Covid-19, it is important that clinicians do not use techniques that they have not used before or been properly trained in and managing the airway of a patient with confirmed or suspected Covid-19 is not the time to test new techniques.1
In conclusion, a number of guidelines regarding airway management of the patient with Covid-19 have recommended the use of video laryngoscopy in preference to direct laryngoscopy for tracheal intubation, providing the operator is competent in the technique, familiar with the device to be used and has the appropriate training.1 In general, single use devices should be used where practical, although a conflict may be created where quality of a single use device is not the same as an alternative reusable option.1 The i-view™ is a single use, fully integrated and disposable video laryngoscope with a Macintosh blade and may be ideal for use where there is a concern regarding infection control, such as during the current pandemic. Any potential user of i-view™ needs to be competent and fully trained with regard to its use, and i-view™ must always be used in accordance with the Instructions For Use supplied with the device.
Information regarding i-view™ and a short instructional video providing an overview of the device and an introduction to the basic steps for correct use can be found at https://www.intersurgical.com/info/iview
References:
- Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75(6):785-799. doi:10.1111/anae.15054
- Yao W, Wang T, Jiang B, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations [published online ahead of print, 2020 Apr 10]. Br J Anaesth. 2020;S0007-0912(20)30203-8. doi:10.1016/j.bja.2020.03.026
- The World Federation of Societies of Anaesthesiologists (WFSA): Coronovirus – guidance for anaesthesia and perioperative providers https://www.wfsahq.org/latest-news/latestnews/943-coronavirus-staying-safe#covid19
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth 2020; 67:568–76
- Zeidan A, Bamadhaj M, Al-Faraidy M, Ali M. Videolaryngoscopy Intubation in Patients with COVID-19: How to Minimize Risk of Aerosolization? [published online ahead of print, 2020 May 20]. Anesthesiology. 2020;10.1097/ALN.0000000000003389. doi:10.1097/ALN.0000000000003389
- European Resuscitation Council Covid-19 Guidelines. Section 3. Advanced Life Support In Adults: https://www.erc.edu/sites/5714e77d5e615861f00f7d18/content_entry5ea884fa4c84867335e4d1ff/5ea8865f4c84867421e4d1eb/files/ERC_covid19_pages_section3.pdf?1588257350
- Guidelines – Infection prevention & control 2020. Association of Anaesthetists. 2020
- i-view video laryngoscope. Intersurgical. https://www.intersurgical.com/info/iview (web-site accessed 17.06.20)
- Gaszynski T. A randomized controlled study on the visual grading of the glottis and the hemodynamics response to laryngoscopy when using I-View and MacGrath Mac videolaryngoscopes in super obese patients [published online ahead of print, 2020 Apr 2]. J Clin Monit Comput. 2020;10.1007/s10877-020-00503-0. doi:10.1007/s10877-020-00503-0